ST. LOUIS – (January 16, 2020) – CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the implantation of over 5,000 3D printed devices using their proprietary Mimetic Metal® technology.
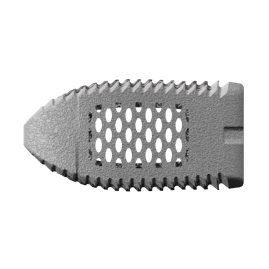
Mimetic Metal is an additively manufactured technology that combines a lattice framework and inner trabecular pores to emulate the structural, functional and physiological properties of bone. The unique dynamic design creates anisotropic stiffness to aid in load transfer. This encourages the structural adaptations of Wolff’s Law, which states that bone density will adapt to functional forces on the bone.
In addition, its open-pore architecture mimics cancellous trabeculae and provides hydrophilic wicking capabilities designed to allow blood flow through the implant. The streamlined titanium alloy (Ti-6AL-4V ELI) support structure also minimizes overall implant density, resulting in beneficial imaging characteristics.
“CoreLink set out to emulate nature with our 3D printed technology. Our unique design has enabled us to optimize everything needed in an interbody device – imaging, graft capacity, porosity, load sharing, strength, and stiffness,” said Jay Bartling, CEO, CoreLink.
“CoreLink set out to emulate nature with our 3D printed technology. Our unique design has enabled us to optimize everything needed in an interbody device – imaging, graft capacity, porosity, load sharing, strength, and stiffness,” said Jay Bartling, CEO, CoreLink. “From the development of the technology to execution of the 3D printing, we design and manufacture Mimetic Metal implants in our facility, ensuring both form and function are met to help patients in their healing process.”
CoreLink has a comprehensive offering of Mimetic Metal implants and exceptionally crafted instrumentation, including the CoreLink M3 Stand-Alone ALIF and F3D family of implants. F3D interbodies include curved, straight, anterior lumbar, cervical, and lateral implants in an array of footprints, heights, and lordosis.
CoreLink surpassed the 5,000th 3D printed device benchmark in November 2019. The first implantation was with the F3D anterior cervical interbody in June 2017.
CoreLink will be exhibiting at the International Society for the Advancement of Spine Surgery (ISASS) Annual Meeting in San Juan, Puerto Rico February 26 – 28, where a full display of surgical systems will be featured.
About CoreLink
CoreLink, known as The Source for Spine™, internally designs and manufactures more than 99% of its broad portfolio of spinal implant systems. With a unique heritage that combines old-world craftsmanship with state-of-the-art manufacturing, we collaborate with surgeons to develop and deliver effective surgical solutions and improve the lives of patients. Learn more at corelinksurgical.com.