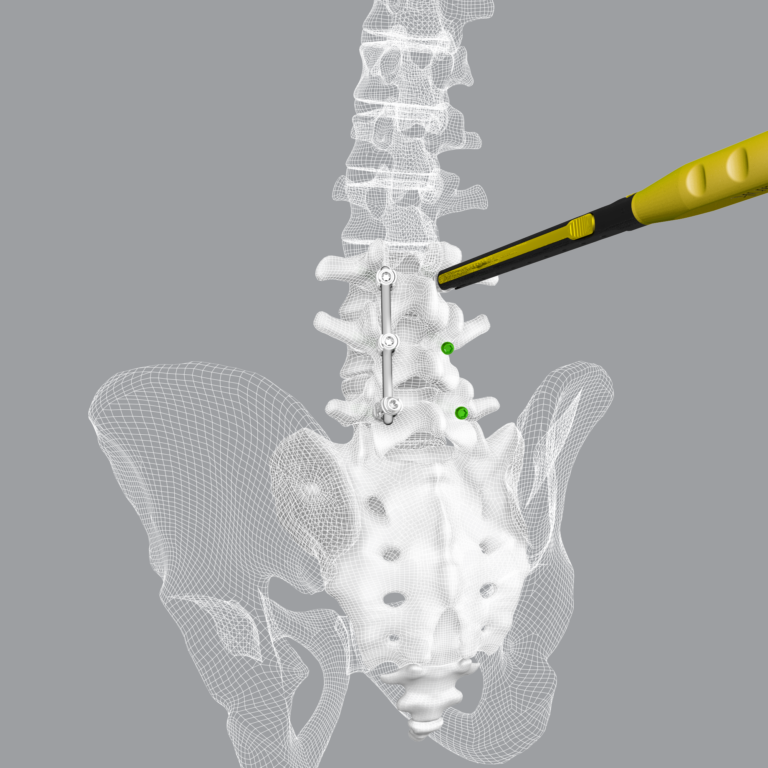
ST. LOUIS–(December 2, 2021)– CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the commercial launch and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the CoreLink CentraFix™ Midline Fixation System.
CentraFix is a posterior thoracolumbar pedicle screw system designed for less invasive spinal fixation often used with a medial-to-lateral approach, known as cortical bone trajectory (CBT). This technique maximizes contact of the pedicle screw with cortical bone and is intended to reduce incision size, limit muscular and vascular injury, and improve initial fixation. CentraFix features modular cobalt chrome tulip heads and titanium alloy screw shanks in various lengths and diameters, designed specifically to allow screw placement in denser cortical bone.
“The CentraFix System provides unmatched intraoperative visualization and surgical flexibility for the midline approach,” said Jay Bartling, CEO of CoreLink. “Our in-house development and manufacturing have allowed us to produce our most innovative fixation system to date.”
“Our in-house development and manufacturing have allowed us to produce our most innovative fixation system to date.”
The CentraFix low-profile, modular tulip heads have been designed to minimize tissue disruption and simplify distraction without compromising strength. Self-drilling, self-tapping cortical screw threading provides easy screw starting in the intended trajectory and allow for 360° motion with a 60° cone of angulation. The system includes 4.75mm and 5.5mm rod options, set screws designed to minimize tulip splay, and a robust offering of ergonomic and intuitive instrumentation to facilitate fast and efficient surgery.