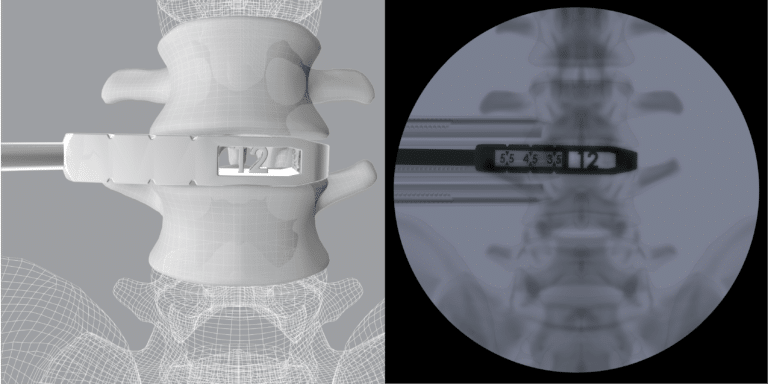
CoreLink OLIF additively manufactured Trials feature height and length measurements visible only under fluoroscopy to facilitate implant selection and operative workflow.
ST. LOUIS–(September 15, 2020)–CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the limited commercial release of the Oblique Lumbar Interbody Fusion (OLIF) Instrument Set. The OLIF technique is a less invasive approach to the lower lumbar spine, which can allow for faster recovery time and minimized psoas disruption.
“The addition of OLIF instrumentation bolsters our minimally invasive spine surgery options and builds on our robust lateral access, fusion, and stabilization platforms. We’ve taken the approach a step further with 3D printed surgical steel instrumentation.”
“The addition of OLIF instrumentation bolsters our minimally invasive spine surgery options and builds on our robust lateral access, fusion, and stabilization platforms. We’ve taken the approach a step further with 3D printed surgical steel instrumentation, our latest foray into additive manufacturing technology. This allows us to build light-weight instruments with features that would not be possible using traditional subtractive methods,” said Jay Bartling, CEO of CoreLink. “We challenged ourselves to a strong year of product development and our team has been consistently delivering.”
The CoreLink OLIF Instrument Set is a comprehensive solution with nearly 100 instruments and 40 procedure-specific tools, including an advanced retractor system. Disc preparation and implantation instruments are oblique-angled to provide easy access to the disc space and limit the need to perform any implant rotation or other instrument maneuvers that may excessively strain surrounding anatomical structures. The set features patent-pending 3D printed trials to allow for rapid interbody sizing. The system is compatible with CoreLink’s CL5 PEEK and F3D titanium alloy lateral interbody cages.