September 18, 2019 08:20 AM Eastern Daylight Time
ST. LOUIS–(BUSINESS WIRE)–CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the introduction of the Oro™ Lateral Plate System designed for optimum versatility to treat patients suffering from degenerative disk disease, spondylolisthesis, trauma, or spinal deformities.
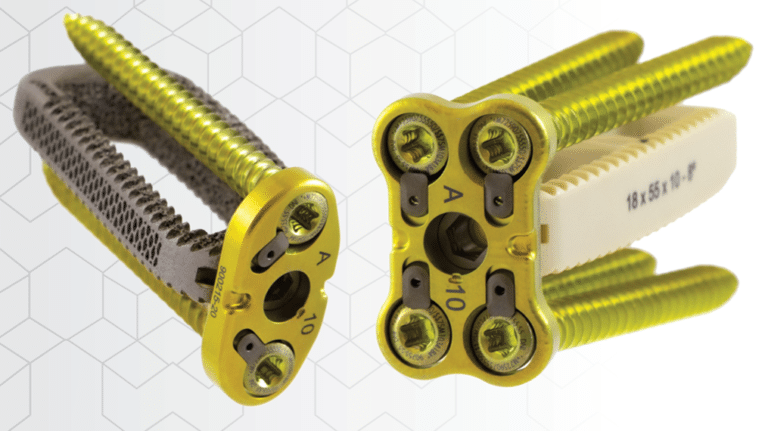
The CoreLink Oro Lateral Plate System has received first of its kind FDA clearance as the only lateral plating system on the market with the flexibility to be used as supplemental fixation in multiple scenarios to allow optional permanent connection to CoreLink CL5 or F3D Lateral interbodies.
“With our continued focus on innovation, this new lateral plate system is the next phase of our lateral offerings and is designed to be tailored to each patient’s needs,” said Jay Bartling, CEO, CoreLink.
“With our continued focus on innovation, this new lateral plate system is the next phase of our lateral offerings and is designed to be tailored to each patient’s needs,” said Jay Bartling, CEO, CoreLink. “This is our third lateral product launch this year, and we are continuing to invest heavily in the lateral procedure via both new product development and surgeon training. Our customers have been impressed by our new training facility and are excited about the additional lateral products in our pipeline.”
The Oro Lateral Plate System, or Oro, is a lumbar supplemental fixation device designed to provide temporary stability until fusion is achieved. This low-profile plate can attach to CoreLink’s CL5 PEEK or F3D lateral interbodies at one or two contiguous levels without the need for additional lumbar fixation, such as pedicle screws. F3D implants leverage the company’s proprietary Mimetic Metal™ technology, a state-of-the-art titanium alloy 3D printing process that emulates key characteristics of natural bone. With a trabecular structure of interconnected pores and a directional lattice perimeter for anisotropic load sharing, Mimetic Metal provides an optimal balance between biomechanical performance and imaging characteristics.
“Patients come to me with the desire for rapid recovery from spinal surgery, and their cases are varied and complex,” said Ryan DenHaese, MS, MD, FAANS. “I prefer to have multiple options on hand in surgery for each different case, and CoreLink’s new lateral plate system gives me flexibility and a single source for exceptional implants and instrumentation. The fact that the company both designs and manufactures these devices provides me the opportunity for individualized surgical instruments and ensures a seamless integration that results in efficient procedures and positive patient outcomes.”
CoreLink received FDA 510(k) clearance for Oro on June 12th, 2019, and its first case was completed shortly after with Dr. Clint Hill in Paducah, KY.
“The Oro Lateral Plate was developed to improve surgical efficiency and provide versatility to its users,” said Dr. Clint Hill. “In my first case using this system and in numerous cases since, I was able to address patient issues due to degenerative disk disease. The ability to attach the plate to the CoreLink interbody in-situ or on the back table facilitates implantation and the flexible sizing allows me the freedom to focus on what’s best for the patient. ”
CoreLink will be exhibiting at the North American Spine Society’s annual meeting in Chicago, September 25-28, booth #2207, where a full display of the lateral surgical systems, including Oro, will be featured.
About CoreLink
CoreLink, known as The Source for Spine™, internally designs and manufactures more than 99% of its broad portfolio of spinal implant systems. With a unique heritage that combines old-world craftsmanship with state-of-the-art manufacturing, we collaborate with surgeons to develop and deliver effective surgical solutions and improve the lives of patients.